Learn
Molar Mass
Now that you've had an introduction to the mole, let's look at the concept of molar mass. Then, we'll apply it to what we have learned about the Law of Conservation of Mass and chemical reactions.
Begin by watching the first 1:21 of Molar Mass and Mole to Gram. PBS login information.
The molar mass is the mass in grams of one mole of a substance – either an atom or a compound. The molar mass of each element is the same as the atomic mass listed on the periodic table.
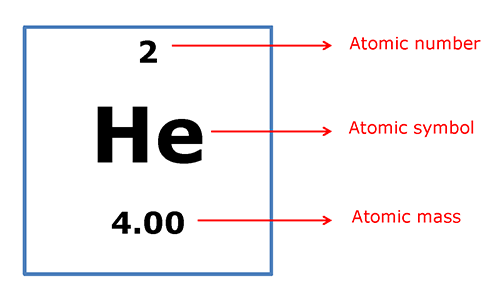
So, 1 mole of helium (He) atoms weighs 4.00g. The atomic mass is also the mass of one atom in atomic mass units (amu). So, 1 atom of helium (He) has a mass of approximately 4.00 amu, and 1 mole of helium (He) atoms has a mass of 4.00 grams.
As you saw in the videos, when you calculate the molar mass of a compound, you add up the molar mass of each individual atom and then add them all together. Let's look at carbon dioxide - CO2.
CO2
Let's start by listing each type of atom and how many of each there are. So there is one atom of C and 2 atoms of O.
C 1
O 2
Then we are going to multiply each one by its individual mass from the periodic table.
C 1 × 12.0g = 12.0g
O 2 × 16.0g = 32.0g
Then we are going to add the totals together to get the total molar mass for the compound.
C 1 × 12.0g = 12.0g
O 2 × 16.0g = 32.0g
12.0g + 32.0g = 44.0g
So the molar mass of CO2 is 44.0g/mol.
For more practice, watch the following. CK-12 login information.
In the next section we will look more at these types of calculations.

