Learn
Ionic Bonding
As you have learned from previous lessons, there are two primary types of atoms: metals and nonmetals. Metals tend to want to lose electrons. Nonmetals tend to want to receive electrons.
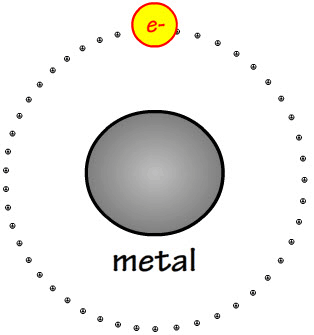
This lone valence electrons on the metal atom here will likely be lost to another element.

This nonmetal atom has 7 valence electrons. In order to complete the octet, it is likely to gain an electron.
When a metal and nonmetal collide into one another and the nonmetal has enough energy to remove an electron from the metal, it does so. Now the two atoms are "stuck together" and ionic bonding has occurred.

These atoms have collided and formed an ionic bond.
Watch Investigate the magnet-like ionic bond formed when electrons transfer from one atom to another (1:00).
Next, let's learn how table salt (NaCl) is formed by ionic bonding!
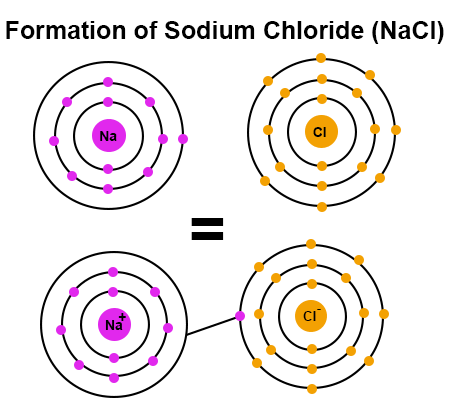
Sodium has one valence electron and chlorine has 7 valence electrons. In order for chlorine to have a stable octet -- or 8 electrons in the outermost energy level -- each chlorine atom has to remove one valence electron from a sodium atom.
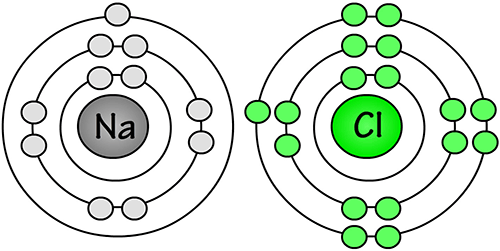
Since each sodium atom has only one valence electron, each one has to give away its only valence electron in order to have a stable octet from a lower energy level.
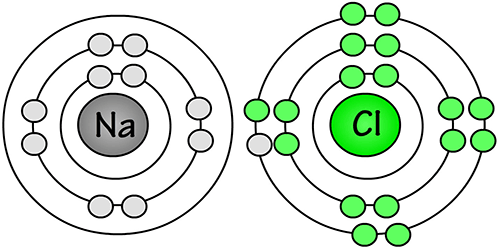
The one valence electron from the sodium (Na) atom is now part of the chlorine (Cl) atom. Now both atoms have formed an ionic bond, and both have stable octets.
It takes 1 chlorine atom to remove 1 valence electron from 1 sodium atom, which forms the compound sodium chloride. This compound NaCl is ordinary table salt!